PGx Drug-Gene Associations
A Cross-Reference of PGx Drug-Gene Association Listings in FDA’s Table of Pharmacogenetic Associations and CPIC Clinical Guidelines
Conducted Quarterly by Manchester University
To help show how perspectives on personalized medicine are evolving at the U.S. Food and Drug Administration and the Clinical Pharmacogenetics Implementation Consortium, which is a leading developer of guidelines in personalized medicine, pharmacogenomics faculty members at Manchester University in Fort Wayne, Indiana, lead a team of Master of Science in Pharmacogenomics students through the following quarterly comparative analysis of FDA and CPIC’s resources regarding drug-gene associations that may have implications for prescribing and dosing decisions. By centralizing information about changes in drug-gene association information published by leading sources, this resource is designed to help researchers, genetic test developers, and health care decision-makers keep up with the latest thinking in the field. For questions or comments about the analysis, please contact Yousif Rojeab using the information provided below.
- Contact: Yousif Rojeab, Ph.D., R.Ph., ybrojeab@manchester.edu
Results: July 22, 2024
According to the most recent cross-reference analysis, 217 drugs are listed with PGx information in FDA’s table, CPIC’s guidelines, or both. The results show that FDA’s table and CPIC’s guidelines overlap through the inclusion of 50 drugs listed by both sources. It is important to note, however, that FDA and CPIC often list differing gene associations and/or dosing implications in connection with those 50 drugs.
Below, you will find an online list of the drugs included in FDA’s table, CPIC’s guidelines, and both; a Venn diagram showing the numbers of drugs included in FDA’s table, CPIC’s guidelines, and both; a downloadable Excel document analyzing the information provided for each of the 50 drugs listed by both sources; and lists of changes made to the drug-gene associations listed in the two sources since March 1, 2022.
DRUGS NAMED BY CPIC, FDA, OR BOTH: AS OF JULY 22, 2024
Abbreviations: CPIC (Clinical Pharmacogenetics Implementation Consortium); FDA (Food and Drug Administration); PK (pharmacokinetics). * Reflects CPIC provisional levels. ** Carbamazepine, codeine, and tramadol are listed in two FDA sections for different gene-drug associations.CPIC Guidelines Only (103 Drugs) CPIC Guidelines and FDA Table (50 Drugs)* FDA Table Only (64 Drugs) - Aceclofenac
- Alfentanil
- Amikacin
- Aminosalicylic acid
- Aspirin
- Atazanavir
- Buprenorphine
- Chloramphenicol
- Chloroquine
- Chlorpropamide
- Ciprofloxacin
- Dabrafenib
- Dapsone
- Desflurane
- Desvenlafaxine
- Dibekacin
- Diclofenac
- Dimercaprol
- Doxorubicin
- Duloxetine
- Enflurane
- Fentanyl
- Fluoxetine
- Fluvastatin
- Furazolidone
- Gentamicin
- Gliclazide
- Glimepiride
- Glipizide
- Glyburide
- Halothane
- Hydrocodone
- Hydromorphone
- Hydroxychloroquine
- Indomethacin
- Isoflurane
- Ivacaftor
- Kanamycin
- Levomethadone
- Levomilnacipran
- Lornoxicam
- Lovastatin
- Lumiracoxib
- Mafenide
- Mepacrine
- Mesalazine
- Metamizole
- Methadone
- Methoxyflurane
- Methylene blue
- Milnacipran
- Morphine
- Moxifloxacin
- Nabumetone
- Nalidixic acid
- Naltrexone
- Naproxen
- Neomycin
- Netilmicin
- Nicorandil
- Nitrofural
- Nitrofurantoin
- Norfloxacin
- Ofloxacin
- Ondansetron
- Oxycodone
- Paromomycin
- Peginterferon alfa-2a
- Peginterferon alfa-2b
- Pegloticase
- Phenazopyridine
- Pitavastatin
- Plazomicin
- Pravastatin
- Primaquine
- Probenecid
- Quinine
- Rasburicase
- Remifentanil
- Ribavirin
- Ribostamycin
- Sertraline
- Sevoflurane
- Sodium nitrite
- Streptomycin
- Sufentanil
- Sulfacetamide
- Sulfadiazine
- Sulfadimidine
- Sulfanilamide
- Sulfisoxazole
- Tafenoquine
- Tegafur
- Tenoxicam
- Tobramycin
- Tolazamide
- Tolbutamide
- Toluidine blue
- Trametinib
- Tropisetron
- Vilazodone
- Vitamin C
- Vitamin K
- Abacavir
- Allopurinol
- Amitriptyline
- Atomoxetine
- Atorvastatin
- Azathioprine
- Capecitabine
- Carbamazepine **
- Celecoxib
- Citalopram
- Clomipramine
- Clopidogrel
- Codeine **
- Desipramine
- Dexlansoprazole
- Doxepin
- Efavirenz
- Escitalopram
- Esomeprazole
- Fluorouracil
- Flurbiprofen
- Fluvoxamine
- Fosphenytoin
- Ibuprofen
- Imipramine
- Lansoprazole
- Meloxicam
- Mercaptopurine
- Nortriptyline
- Omeprazole
- Oxcarbazepine
- Pantoprazole
- Paroxetine
- Phenytoin
- Piroxicam
- Rabeprazole
- Rosuvastatin
- Simvastatin
- Succinylcholine
- Sulfamethoxazole/Trimethoprim
- Sulfasalazine
- Tacrolimus
- Tamoxifen
- Thioguanine
- Tramadol **
- Trimipramine
- Venlafaxine
- Voriconazole
- Vortioxetine
- Warfarin
- Abrocitinib
- Amifampridine
- Amifampridine phosphate
- Amoxapine
- Amphetamine
- Aripiprazole
- Aripiprazole lauroxil
- Avatrombopag
- Belinostat
- Belzutifan
- Brexpiprazole
- Brivaracetam
- Carisoprodol
- Carvedilol
- Cevimeline
- Clobazam
- Clozapine
- Darifenacin
- Deutetrabenazine
- Diazepam
- Dolutegravir
- Donepezil
- Dronabinol
- Elagolix
- Eliglustat
- Erdafitinib
- Fesoterodine
- Flibanserin
- Galantamine
- Gefitinib
- Hydralazine
- Iloperidone
- Irinotecan
- Isoniazid
- Lapatinib
- Lofexidine
- Mavacamten
- Meclizine
- Metoclopramide
- Metoprolol
- Mirabegron
- Mivacurium
- Nateglinide
- Nebivolol
- Nilotinib
- Oliceridine
- Pazopanib
- Perphenazine
- Pimozide
- Pitolisant
- Procainamide
- Propafenone
- Propranolol
- Protriptyline
- Raltegravir
- Risperidone
- Sacituzumab
- Siponimod
- Tamsulosin
- Tetrabenazine
- Thioridazine
- Tolterodine
- Valbenazine
- Viloxazine
WHERE DRUGS NAMED BY FDA AND CPIC APPEAR: AS OF JULY 22, 2024
Drugs Included in FDA's Table of Pharmacogenetic Associations and CPIC Clinical Guidelines: As of July 22, 2024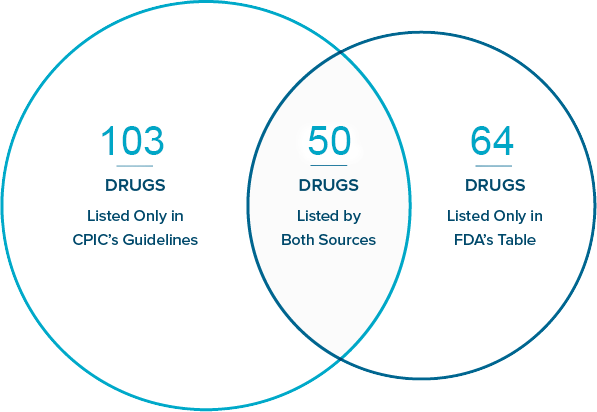
CHANGE LOG: MARCH 28 - JUNE 30, 2023
Drug From: To: Desvenlafaxine Not listed by either source. CPIC's guidelines only. Duloxetine Not listed by either source. CPIC's guidelines only. Fluoxetine Not listed by either source. CPIC's guidelines only. Levomilnacipran Not listed by either source. CPIC's guidelines only. Milnacipran Not listed by either source. CPIC's guidelines only. Vilazodone Not listed by either source. CPIC's guidelines only. Venlafaxine FDA's table only. CPIC's guidelines and FDA's table. Vortioxetine FDA's table only. CPIC's guidelines and FDA's table. CHANGE LOG: JANUARY 4, 2023 - MARCH 28, 2023
Drug From: To: Dibekacin Not listed by either source. CPIC's guidelines only. Neomycin Not listed by either source. CPIC's guidelines only. Netilmicin Not listed by either source. CPIC's guidelines only. Ribostamycin Not listed by either source. CPIC's guidelines only. CHANGE LOG: SEPTEMBER 28, 2022 - JANUARY 4, 2023
Drug From: To: Trimipramine CPIC's guidelines only. CPIC's guidelines and FDA's table. Abrocitinib Not listed by either source. FDA's table only. Nateglinide Not listed by either source. FDA's table only. Mavacamten Not listed by either source. FDA's table only. CHANGE LOG: JULY 8, 2022 - SEPTEMBER 28, 2022
Drug From: To: Tramadol CPIC's guidelines and FDA's table. Updated/additional entry in CPIC's guidelines and FDA's table under "Potential Impact on Safety/Response." Esomeprazole FDA's table only. CPIC's guidelines and FDA's table. Rabeprazole FDA's table only. CPIC's guidelines and FDA's table. Sulfamethoxazole/trimethoprim FDA's table only. CPIC's guidelines and FDA's table. Sulfasalazine FDA's table only. CPIC's guidelines and FDA's table. Aceclofenac Not listed by either source. CPIC's guidelines only. Alfentanil Not listed by either source. CPIC's guidelines only. Aminosalicylic acid Not listed by either source. CPIC's guidelines only. Aspirin Not listed by either source. CPIC's guidelines only. Buprenorphine Not listed by either source. CPIC's guidelines only. Chloramphenicol Not listed by either source. CPIC's guidelines only. Chloroquine Not listed by either source. CPIC's guidelines only. Chlorpropamide Not listed by either source. CPIC's guidelines only. Ciprofloxacin Not listed by either source. CPIC's guidelines only. Dabrafenib Not listed by either source. CPIC's guidelines only. Dapsone Not listed by either source. CPIC's guidelines only. Dicolfenac Not listed by either source. CPIC's guidelines only. Dimercaprol Not listed by either source. CPIC's guidelines only. Doxorubicin Not listed by either source. CPIC's guidelines only. Fentanyl Not listed by either source. CPIC's guidelines only. Furazolidone Not listed by either source. CPIC's guidelines only. Gliclazide Not listed by either source. CPIC's guidelines only. Glimepiride Not listed by either source. CPIC's guidelines only. Glipizide Not listed by either source. CPIC's guidelines only. Glyburide Not listed by either source. CPIC's guidelines only. Hydromorphone Not listed by either source. CPIC's guidelines only. Hydroxychloroquine Not listed by either source. CPIC's guidelines only. Indomethacin Not listed by either source. CPIC's guidelines only. Levomethadone Not listed by either source. CPIC's guidelines only. Lumiracoxib Not listed by either source. CPIC's guidelines only. Mafenide Not listed by either source. CPIC's guidelines only. Mepacrine Not listed by either source. CPIC's guidelines only. Mesalazine Not listed by either source. CPIC's guidelines only. Metamizole Not listed by either source. CPIC's guidelines only. Methadone Not listed by either source. CPIC's guidelines only. Methylene blue Not listed by either source. CPIC's guidelines only. Morphine Not listed by either source. CPIC's guidelines only. Moxifloxacin Not listed by either source. CPIC's guidelines only. Nabumetone Not listed by either source. CPIC's guidelines only. Nalidixic acid Not listed by either source. CPIC's guidelines only. Naltrexone Not listed by either source. CPIC's guidelines only. Naproxen Not listed by either source. CPIC's guidelines only. Nicorandil Not listed by either source. CPIC's guidelines only. Nitrofural Not listed by either source. CPIC's guidelines only. Nitrofurantoin Not listed by either source. CPIC's guidelines only. Norfloxacin Not listed by either source. CPIC's guidelines only. Ofloxacin Not listed by either source. CPIC's guidelines only. Oxycodone Not listed by either source. CPIC's guidelines only. Pegloticase Not listed by either source. CPIC's guidelines only. Phenazopyridine Not listed by either source. CPIC's guidelines only. Primaquine Not listed by either source. CPIC's guidelines only. Probenecid Not listed by either source. CPIC's guidelines only. Quinine Not listed by either source. CPIC's guidelines only. Rasburicase Not listed by either source. CPIC's guidelines only. Remifentanil Not listed by either source. CPIC's guidelines only. Ribavirin Not listed by either source. CPIC's guidelines only. Sodium nitrite Not listed by either source. CPIC's guidelines only. Sufentanil Not listed by either source. CPIC's guidelines only. Sulfacetamide Not listed by either source. CPIC's guidelines only. Sulfadiazine Not listed by either source. CPIC's guidelines only. Sulfadimidine Not listed by either source. CPIC's guidelines only. Sulfanilamide Not listed by either source. CPIC's guidelines only. Sulfisoxazole Not listed by either source. CPIC's guidelines only. Tafenoquine Not listed by either source. CPIC's guidelines only. Tegafur Not listed by either source. CPIC's guidelines only. Tolazamide Not listed by either source. CPIC's guidelines only. Tolbutamide Not listed by either source. CPIC's guidelines only. Toluidine blue Not listed by either source. CPIC's guidelines only. Trametinib Not listed by either source. CPIC's guidelines only. Vitamin C Not listed by either source. CPIC's guidelines only. Vitamin K Not listed by either source. CPIC's guidelines only. CHANGE LOG: APRIL 6, 2022 - JULY 8, 2022
Drug From: To: Amikacin Not listed by either source. CPIC's guidelines only. Gentamicin Not listed by either source. CPIC's guidelines only. Kanamycin Not listed by either source. CPIC's guidelines only. Paromomycin Not listed by either source. CPIC's guidelines only. Plazomicin Not listed by either source. CPIC's guidelines only. Streptomycin Not listed by either source. CPIC's guidelines only. Tobramycin Not listed by either source. CPIC's guidelines only. Fluvastatin Not listed by either source. CPIC's guidelines only. Lovastatin Not listed by either source. CPIC's guidelines only. Pitavastatin Not listed by either source. CPIC's guidelines only. Pravastatin Not listed by either source. CPIC's guidelines only. Belzutifan Not listed by either source. FDA's table only. Lansoprazole CPIC's guidelines only. CPIC's guidelines and FDA's table. CHANGE LOG: MARCH 1, 2022 - APRIL 6, 2022
Drug From: To: Fosphenytoin CPIC's guidelines only. CPIC's guidelines and FDA's table. Phenytoin CPIC's guidelines only. CPIC's guidelines and FDA's table. Atorvastatin FDA's table only. CPIC's guidelines and FDA's table. Rosuvastatin FDA's table only. CPIC's guidelines and FDA's table. Viloxazine Not listed by either source. FDA's table only. Biomarker From: To: NUDT15 PharmGKB 2B PharmGKB 1A Drug-Biomarker From: To: Succinylcholine-BCHE CPIC C/D CPIC B/C Tramadol-CYP2D6 PharmGKB 1B PharmGKB 1A